4 Development & Changes of the Skeletal System
“To succeed in life, you need three
things: a wishbone, a backbone
and a funny bone”.
-Reba McEntire
The skeletal system provides the architectural support and structure of the body, while at the same time it allows for movement, and the protection of internal organs. The skeletal system also serves as a repository for minerals which are needed to maintain normal functioning. In particular, calcium, phosphate and other ions are stored in bone as crystalline salts. These salts contribute to the bone’s strength and assists with the bone’s ability to withstand compressive forces of weight bearing. The stored minerals are also used to maintain blood mineral levels when changes in diet or metabolic demands occur. In addition, bone marrow, which is important in the production of blood cells is stored in bone.
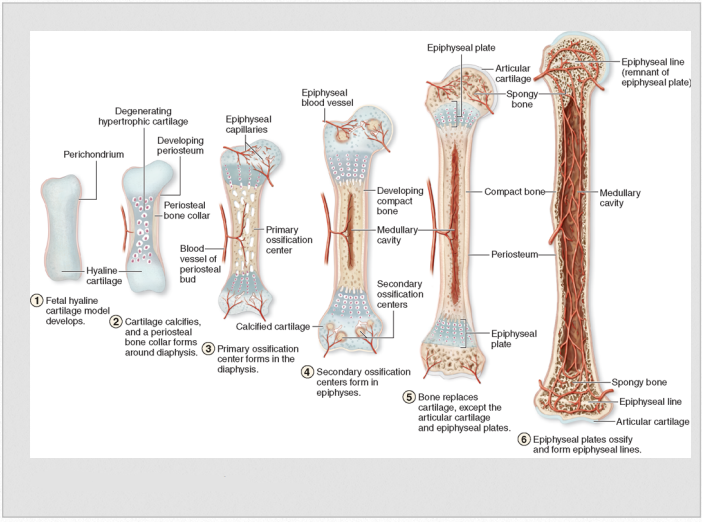
4.1 Endocondral Ossification
Bone is a connective tissue composed of bone cells and bone matrix held together by a ground substance. The bone matrix is a hard, calcified substance made up of collagen fibers and mineral salts. The matrix surrounds the osteocytes, which are responsible for maintaining the nutrition and mineral content of the bone matrix. The osteoblasts are bone cells that are active in the formation of new bone, whereas the osteoclasts are associated with resorption of bone.
All bones are made up of two types of bone tissue. Compact bone, also called cortical bone, is hard and dense and makes up the majority of bones in the body. Cancellous bone, also called trabecular or spongy bone, is comprised of loosely woven strands of bone tissue and represents only 20% of bones in skeleton. Compact bone makes up the shaft of long bones and is resistance to bending and torsional forces. Cancellous bone, houses the bone marrow, minerals and vessels that nourish the bone and allows for more flexibility than compact
bone, making it more responsive to compressive forces (Walker, 2020).
In addition to bones, the skeletal system includes cartilage, joints, ligaments, tendons and other connective tissues and when attached to muscles, allow for movement.
Prenatal
In utero, skeletal development begins with cartilage, which is derived from the embryonic mesoderm. Early in fetal development the skeleton is largely cartilaginous, which either remains unossified throughout life or is replaced by bone. Cartilage that does not go through the process of ossification form the ears and nose, and can be found at the ribs and joints.
Development of bone occurs either through intramembraneous or endochondral ossification and begins in the 3rd – 8th weeks of gestational age. In the 6th week mesenchymal cells have differentiated into chondroblasts, which form the cartilage model of long bones. Then, primary centers of ossification centers begin to appear so that by week 12, ossifcation centers appear in almost all the bone of the extremities. In long bones, primary ossification centers are located in the diaphysis or shaft of the bone. A few secondary ossification centers appear late in fetal development and are found at the ends of long bone in an area called the epiphyses. The diaphyses are fairly well ossified by birth, but the epiphyses remain cartilaginous (Breeland, et al, 2021). Intramembranous ossification takes place directly within mesenchyme tissue, such that the mesenchymal cells don’t differentiate to cartilage first. In this process, numerous ossification centers are formed in the fibrous connective tissue membrane which fuse into cancellous or spongy bone tissue. In time, this will become compact bone. The bones of the skull are formed in this way. Functionally, intramembranous ossification of the skull helps to protect the developing brain. However, because the skull is not fused, as evidenced by the fontanells, expansion and molding of the cranium can respond to brain growth while at the same time allow for passage through the birth canal.
[Alternative Image Required]
Figure 4.2 Intramembranous bone formation
After the early models of bone are present, joint formation occurs, and by the early fetal period, most joints have formed. In articular joints, mesenchymal cells differentiate into the joint capsule, ligaments, tendons and menisci. Once the joint is formed, intrauterine movement is important for ongoing joint development.
If the intrauterine environment is too confining, the skeletal system is at risk for developing deformities such as congenital hip dislocation, tibial bowing, metatarsus adductus, and calcaneus varus. Although many deformities will improve within the first few years of life, others, such as hip dysplasia may require orthopedic management (Cech & Martin,
2012; Walker, 1991).
Infancy and Childhood
Rapid bone growth occurs during infancy and childhood which affects not only bone length, but diameter as well. The process, in which new bone is laid down on the outer surface of the bone and absorbed from the inner surface, is called appositional growth. This type of growth occurs throughout life, although the ratio of bone formation and bone resorption varies. During infancy and childhood and through the better part of adolescence, formation is greater than resorption. This process can be positively influenced by mechanical stress such as
weight-bearing and functional activities. Appositional bone growth also allows for re-shaping of the bone as it grows in length.
Another process of bone development occurs at the traction epiphyses, a secondary center of ossification, located at the attachment site of a tendon. Bone is developed as a response to the amount and direction of muscular contraction that occurs at this site (Farr, 2015). In addition to weight bearing and muscle contraction, nutrition and hormones play important roles in the development and maintenance of bone. Infants and children who do not have sufficient protein, calcium, vitamins C and D in their diets experience abnormal bone growth. Appropriate levels of growth and thyroid hormones are critical for normal bone development. Later, lower levels of testosterone, estrogen and growth hormone (GH) are associated with bone loss in older adults.
Besides an increase in the size of bone, the human skeleton changes in shape, rotation, angulation, and proportions during infancy and childhood. For example, a newborn’s head and trunk make up a proportionality larger part of the the skeleton than it does during late childhood. As the infant begins to move through crawling, standing, and walking, the lower extremities pelvis, and spine undergo angular and rotational changes with the curvature of the sacrum and the acetabular depth increasing. Even before that though, changes of the spine occur as the infant develops head control and reveals cervical lordosis. Developing the ability to sit contributes to the development of the lordotic curve of the lumbar spine (Cech & Martin, 2012).
Changes of the proximal femur including the femoral neck and it’s decreasing angle of inclination
creates a better lever arm for the action of hip abduction. During infancy, the angle between the
femoral neck and the shaft measures between 135-145 decrees whereas the angle of inclination is 125 degrees in the average adult (Figure 4.4). The femoral angle of torsion also changes during childhood, such that the amount of anteversion decreases from birth to adulthood.
Angular and torsional changes also occur at the tibia and ankle/foot couple. The relationship between the femur and the tibia changes from a position of genu varum during infancy to genu valgus by age three. There is also a change in tibial torsion that occurs overtime. At birth, tibial torsion is typically between 5-10 degrees of internal rotation. Most adults however, have tibial torsion of 20-25 degrees of external rotation. Alignment at the calcaneus also changes from birth to adulthood. Newborns have a calcaneal position of approximately 20 degrees varus, which through lower extremity weight bearing, is reduced to just a couple of degrees over time (Bernhardt, 1988).
Skeletal System Concerns
During infancy and childhood the epiphysis is an active site for new bone formation, playing an important role in skeletal development. Newborns are particularly vulnerable to infection of the epiphysis because the epiphyseal plate is very thin.

This allows blood vessels to easily pass through, allowing infection to easily spread. As the epiphyseal plate thickens, the blood vessels cannot pass through thereby eliminating the risk of infection.
During childhood fractures of the epiphyseal or growth plate can be seen because the plate is
largely cartilaginous and not as resistant to stress as ossified bone. Although many growth plate fractures heal well, fractures at this site put children at risk for abnormal bone growth which can lead to limb length discrepancies and joint abnormalities (Figure 4.5)
Even though bone mineral density is increasing during infancy and childhood through the process of appositional growth, children’s bones are less dense and more porous than adult bones, which
make them sensitive to compressive and tensile stress. This vulnerability makes them susceptible to greenstick fractures and apophyseal avulsion. With a greenstick fracture, the bone does not break all the way through (Figure 4.5) With an apophyseal avulsion, which can be referred to as an avulsion fracture, a sudden, forceful muscle contraction causes a fracture by pulling a piece of bone away at its insertion site (Walker, 1991).
Adolescence
During adolescence, bone continues to grow and remodel. This process continues to be influenced by size, physical activity, and nutritional intake, all of which effects the bone matrix and mineralization. In particular, calcium absorption is most efficient during puberty.

Figure 4.4 Epiphyseal and Greenstick Fracture
The adolescent growth spurt among girls begins on average at 12 years of age, whereas boys typically have their growth spurt beginning at 14 years of age. This is in part due to an increase in androgens and estrogen, which act to support bone growth in addition to sexual development. In addition to stimulating appositional growth and increased cortical bone density, it appears that the androgens and estrogen may stimulate cancellous bone development which is more apparent in later puberty. High-impact physical activity has been found to increase site-specific bone mineral density in early puberty and adolescence (Stope, 2015).
On average, boys have high growth periods longer than girls. Regardless of sex though, this rapid
change in bone growth often outpaces increases in muscle length. This can result in decreased flexibility and should be kept in mind during exercise and other types of physical activities. Recommendations for stretching may be warranted in an effort to modify risk of discomfort and injury.
Although bone growth usually ceases before the epiphyses close, the term skeletal maturity refers to that time of epiphyseal closure. This occurs earlier in females than in males and is usually complete during the early 20s. When skeletal maturity is reached 95% of peak bone mass is present, the remaining 5% is attained through appositional growth (Cech & Martin, 2012).
Skeletal System Concerns
There are a few skeletal problems that can arise, in part due to the rapid growth that occurs during adolescence. Teenagers, for example, are at the greatest risk for developing irritation of the apopysis or traction epiphysis of the tibial plateau. This is where the patellar tendon attaches to the tibia. It is suspected that rapid bone growth will cause the tendon to pull on the bone, resulting in apophysitis, also known a Osgood-Schlatter disease. Decreasing the eccentric work of the quadriceps while carefully increasing the quadriceps length can be helpful in addressing this condition while at the same prevent additional knee joint pathology (Smith & Varacallo, 2021).
Rapid growth during adolescence also increases the vulnerability of the open epiphysis and increases the risk of stress and avulsion fractures especially in athletes who have their training levels increased. Common sites for growth plate injury and stress fractures are the proximal humerus, distal radius, lumbar spine, tibia and fibula. The most common stress fracture of the spine is seen most often in gymnasts and wrestlers. It occurs at the pars interarticularis of the vertebra and is called spondylolysis (Standaert & Herring, 2000).
Scoliosis, which is a curvature of the spine, is another spinal condition that is typically first identified during late childhood or adolescence. Mild scoliosis of 5 degrees or less is seen in 10% of children during puberty. Although only a small percentage of scoliotic curves progress to more than 15 degrees, more dramatic curves can have serious affects on postural alignment, muscle length, movement resulting in pain and functional limitation (Konieczny, et al, 2013).
The risk of avulsion fractures is noted in children, but continues to be a risk in adolescents, particularly those involved in competitive sports. Common sites for avulsion fractures in teenage are the anterior super iliac spine, the anterior inferior iliac spine, the lesser trochanter and the ischium.
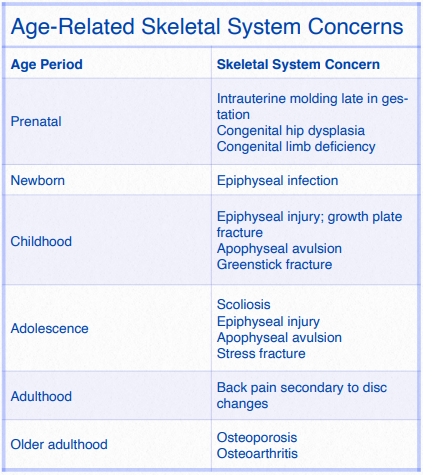
Figure 4.5 Age-Related Skeletal System Concerns
Adults and Older Adults
During adulthood, bones do not get longer, but remodeling does occur, stimulated by weight bearing, muscle contraction, and proper nutrition. Both sexes attain maximal bone mass by about 30 years of age. From approximately 30 – 50 years of age, bone formation and resorption are essentially equal, so that bone mass remains constant. After that, bone resorption surpasses bone formation. Cancellous bone loss is observed to precede cortical loss. During adulthood men and women lose from 0.5% to 1% of bone mass per year, although this rate fluctuates in women. During the 4-8 years after menopause, bone loss can be accelerated to a loss rate of 2%, but after time the rate of loss returns to 0.5 – 1% (Farr, 201300.)
Over time, the skeletal system becomes even more compromised with bone loss continuing into older adulthood. This may be due to decreased level of circulating hormones and dietary changes, along with a decline in physical activity. Diets rich in calcium and vitamin D, along with strength and power training, have been demonstrated to be helpful in reducing the rate of bone loss.
Skeletal System Concerns
Unfortunately, the bone loss that occurs with normal aging puts older adults at risk for osteoporosis, a condition in which a person’s bone mineral density is equal to or more than 2.5 standard deviations below a normal reference range. Bones of the spine, wrist and hip are particularly susceptible to bone loss because these areas are made up of a high proportion of cancellous bone. During the aging process, cancellous bone loss is known to occur earlier than cortical bone loss.
It is suspected that 50% of women and 25% of men have osteoporosis, which can lead to impaired posture, pain, limited mobility, and fractures. In older adulthood, these conditions have been associated with feelings of isolation, depression, and the need for long-term care.
Changes to the skeletal system during the aging process also includes alteration to cartilage and
joints. Cartilage consists of water, proteoglycans, collagen fibers, and chondrocytes. The proteoglycans and collagen fibers make up the extracellular collagen matrix. Differences in the matrix and the amount of water in the tissue help to differentiate articular cartilage from fibrocartilage. Articular cartilage provides a low friction surface for easy joint movement and is comprised of 60%-80% water. Fibrocartilage, which is found at the intervertebral discs, menisci and tendinous insertions is comprise of 50% water. Cartilage has no nerve or vascular supply on its own and must obtain oxygen and nutrition from surrounding tissues. Mechanical loading and unloading of articular cartilage is critical for its ability to maintain nutrition and remain healthy.
During the aging process, the composition of articular cartilage changes due to 1) alterations in the cellular matrix, 2) a less effective chondrocyte repair process, 3) cross-linkage of protein and collagen, 4) increased calcification of cartilage, 4) loss of water concentration, and 5) increased fibrillation (Roberts, et al, 2016).
The matrix changes account for water and protein losses that contribute to stiffness and resilience of articular cartilage. As it loses its stiffness and tensile strength, the articular cartilage becomes more susceptible to injury because it cannot withstand the stresses placed upon it.
These articular cartilage changes can lead to impairments in weight-bearing and movement which
can lead to osteoarthritis. Affecting nearly 70% of all older adults, this pathological condition can
cause pain, impaired range of motion, and loss of physical functioning (Loeser, 2010).
Final Thoughts
The skeletal system develops and changes over a life time and provides a structural framework from which movement and functional activities can occur. Optimal development of the skeletal system not only requires proper nutrition, it requires active movement and weight bearing to help achieve and maintain its most efficient form.
References
Bernhardt, D.B. (1988). Prenatal and postnatal growth of development of the foot and ankle. Phys Ther, 68(12), 1831-1839.
Breeland G, Sinkler MA, Menezes RG. Embryology, Bone Ossification. [Updated 2021 May 8]. In: StatPearls [Internet].
Treasure Island (FL): StatPearls Publishing; 2022 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK539718/
Cech, D. J. & Martin, S. T. (2012). Skeletal System Changes. In D.J. Cech, & S.T. Martin (Eds.), Functional Movement Development Across the Life Span. St. Louis, MO, Elsevier.
Farr, J.N. & Khosla, S. (2015). Skeletal changes through the
lifespan – from birth to senescence. National Review of Endocrinology, 11(9), 513-531.
Konieczny, M.R., Senyurt, H., & Krauspe, R.(2013). Epidemiology of adolescent idiopathic scoliosis. Journal of Childhood Orthopedics, 7, 3-9.
Loeser R. F. (2010). Age-related changes in the musculoskeletal system and the development of osteoarthritis.
Clinics in Geriatric Medicine, 26(3), 371–386. https://doi.org/10.1016/j.cger.2010.03.002
Roberts, S., Colombier, P., Sowman, A., Mennan, C., Rölfing, J. H., Guicheux, J., & Edwards, J. R. (2016). Ageing in the musculoskeletal system. Acta Orthopaedica, 87(sup363), 15–25. https://doi.org/10.1080/17453674.2016.1244750
Smith, J.M., & Varacallo, M. Osgood Schlatter Disease. [Updated 2021 Jul 30]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK441995/
Standaert C.J., & Herring, S.A. (2000). Spondylolysis: a critical review British Journal of Sports Medicine 34, 415-422.
Strope, M.A. et al (2015). Physical activity-associated bone loading during adolescence and young adulthood is positively associated with adult bone mineral density in men. American Journal of Men’s Health, 9(6), 442-450.
Walker, J. (2020). Skeletal system 1: The anatomy and physiology of bones. Nursing Times [online]; 116: 2, 38-42.
Walker J. M. (1991). Musculoskeletal development: a review. Physical Therapy, 71(12), 878–889. https://doi.org/10.1093/ptj/71.12.878
Images
Title image. [Image File]. Retrieved from https://pixabay.com/en/skeleton-bones-skeleton-sections-2277446/
Figure 4.1 Endochondral Ossification. [Image File]. Retrieved from https://www.researchgate.net/post/Does-anyoneknow-about-compact-bone-formation-during-Endochondral-Ossification
Figure 4.2 Intramembranous bone formation. [Image File]. Retrieved from http://highered.mheducation.com/sites/dl/free/0072507470/234420/mc_ch06_fig08.jpg
Figure 4.3 Normal Mean Angle of Femoral Neck-Shaft According to Age from Hung, NN (2013). Congenital dislocation of the hip in children between the ages of one and three: Open reduction and modified salter innominate osteotomy combined with fibular allograft, Open Journal of Orthopedics, 3(2), 137-152.
Figure 4.4 Epiphyseal and Greenstick Fracture. [Image File]. Retrieved from https://medlineplus.gov/ency/images/ency/fullsize/18022.jpg
Figure 4.5 Age-Related Skeletal System Concerns. Cech, D. J. & Martin, S. T. (2012). Skeletal System Changes. In D.J. Cech, & S.T. Martin (Eds.), Functional Movement Development Across the Life Span. St. Louis, MO, Elsevier.

