6 Development & Changes of the Nervous and Sensory Systems
Development & Changes of
the Nervous and Sensory
Systems
“God may forgive your sins, but your nervous system won’t.”
– Alfred Korzbski
Nervous System
The nervous system is often considered to be the hard drive or command center for all human function. It is comprised of the brain, spinal cord and a large network of nerves that connect the body to the brain. Together, the brain and spinal cord comprise the central nervous system (CNS). The large network of nerves found throughout the body is known as the peripheral nervous system (PNS).
The CNS and PNS work together to receive, process, integrate and respond to 1) physiologic processes within the body and 2) environmental stimuli and inputs. The nervous system is an overseer of all the other bodily systems and is responsible for their integration during all the major functions of moving, thinking, and feeling (SEER, 2022)
Prenatal
The CNS begins to develop at approximately three weeks gestation. The neural ectoderm gives rise to the neural plate, and then the neural folds. From the neural folds, the neural tube and CNS develops. The brain is created from the superior (cranial) twothirds of the tube, whereas the spinal cord develops from the distal (caudal) two-thirds (Shiota, 2015).
In addition to giving rise to the neural tube, the neural plate gives rise to neural crest cells, which are the precursors of the PNS. The PNS begins as paired masses of neural crest cells, one on each side of the neural tube, that differentiate into the sensory ganglia of the spinal nerves (Figure 6.1)
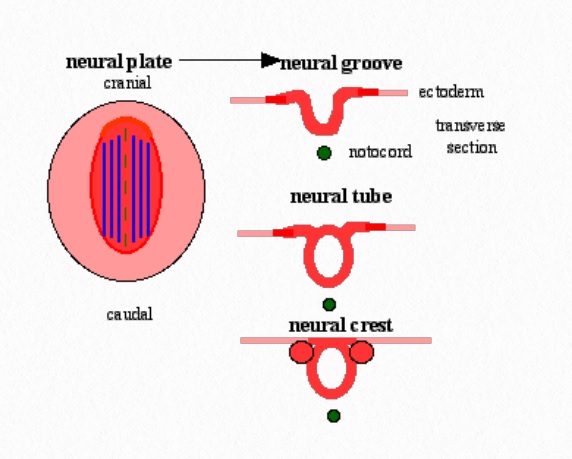
Figure 6.1 Neural Plate
The neural crest cells in the brain region migrate to form sensory ganglia for cranial nerves V, VII, VIII, IX, and X. Other structures are also produced from the neural crest cells: Schwann cells, meninges (the connective tissue covering of the brain), and many musculoskeletal components of the head.
As neurons develop and differentiate they travel to a final destination. Some form the brain stem which will end up controlling the heart and lungs, some will end in the cerebellum, which will help control posture and balance and some will migrate to the cerebral cortex were perception and thought occur. Once the neurons are in place, they grow an axon
which allows for synapses to occur. Initially, the synapses occur in highly variable patterns of distribution. With development, there is a pruning of neurons, their branches, and connections, so that neural networks can become efficient.
The development of connections between sensory neurons and motor neurons is critical for the pairing of sensory and motor information. This connection also forms the foundation of spinal reflexes, which allows incoming stimuli to produce a motor response. Once established, spinal reflexes are considered “hard-wired”. In utero, movement driven by reflexes have been observed as early as seven weeks (Fagard, et al. 2018).
Teratogens are drugs, chemicals or infections that can cause abnormal fetal development and can affect an embryo as early as 10 to 14 days after conception. During the prenatal period, the nervous system is particularly susceptible to teratogenic exposure, including nicotine, alcohol, drugs and infections such as rubella, cytomegalovirus (CMV) and toxoplasmosis, which can be transmitted from cat feces. Exposure to any of the conditions can have negative effects on the developing nervous system which can result in birth defects and intellectual
disabilities. Although not a teratogen, malnutrition, can also create developmental challenges with long-standing effects (Tantibanchachai, 2014).
Infancy and Early Childhood
At birth, the brain is about 25% of its adult weight and at six months, its weight has doubled. During infancy, critical periods of brain growth occur between three and ten months and again between fifteen and 24 months. By the time children are four years old, their brains have reached 80% of adult weight. This rapid growth reflects an increase in the size of the neurons, an increase in the number of neuronal branches and synapse, and an increase inglia and myelin. Glia are the cells of the nervous system that support and nourish the neurons.
Myelin is an insulating sheath found around the axons of many nerves, which help to maintain the functions of the nervous system by increasing the conduction speed of impulses from the cell body to receiving neurons, glands and muscles (Cech & Martin, 2012).
Brain metabolism changes as the CNS matures during infancy, such that areas of the brain with increased glucose metabolism correspond with areas of the brain that are maturing. In the neonate, the highest activity is found in the primary motor and sensory cortex, thalamus, brain stem and midline of the cerebellum. By two-three months, increases in glucose utilization are evident in the parietal, temporal and primary visual cortex, basal ganglia and cerebellar hemispheres.
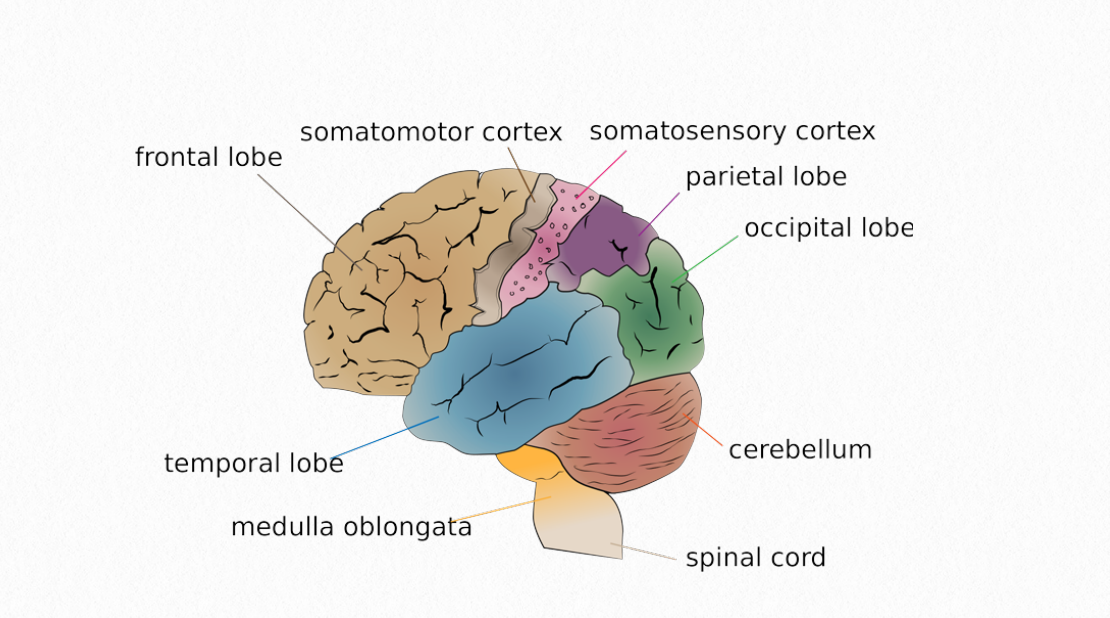
Figure 6.2 Human Brain
The frontal lobe is the last area is the last area to demonstrate an increased use of glucose. This corresponds with an increase in dendritic branches and an expanding capillary network, which begins between six-eight months (Chugani, 2018).
Myelination is also occurring in the newborn’s brain and will continue into young adulthood. In general, brain development progresses from posterior to anterior and from interior to superior, with myelination of the cerebellum and the brain stem occurring before the cerebral hemispheres. The first two months after birth are considered to be a period of organization for the CNS. During this time the physiological control of sleep and wakefulness is occurring and the circadian rhythm is being established.
In addition, the corticospinal tract, which is the major efferent tact, begins myelination approximately one month before birth. In the neonate, the corticospinal tract has not yet innervated the anterior horn, so during the first six months, the infant will demonstrate the Babinski sign when the bottom of the foot is stroked. There appears to be a correlation between late corticospinal development and late motor development. The sensory area of the brain catches up to the motor area by the by the age of two. During the second year of life, the increasing speed and complexity of movement that’s observed among this age group may be related to myelination. The process slows after two years and is mostly complete by ten years of age (Welniarz, et al 2017).
Nerve conduction velocity increases over time in both skin and muscle fibers because of a change in saltatory conduction and the increase in nerve fiber diameter with age. Values for nerve conduction speed change remarkably quickly after birth. For example, ulnar nerve conduction in infants and young children increases from 30 to 50 m/sec from birth to nine months of age and reaches adult values (60 m/sec) by three years of age (VecchieriniBlineaus & Guiheneuc, 1984).

Figure 6.3 Children Jumping
Late Childhood and Adolescence
Although the increase in speed of nerve conduction is positive, it doesn’t mean that young children are able to perform high level proficient motor activities. This is because the corticospinal tract does not achieve electrophysiological maturity until about thirteen years of age. Nevertheless, children ages three to six are developing fundamental motor skills such as throwing, catching, jumping and balancing. Then, along with ongoing myelination of the nervous system, particularly the corticospinal tract, these skills become much more refined (Welniarz, et al 2017).The adolescent will be able to further advance these skills and others with proper instruction, motivation and practice.
Myelination continues during adolescence in the sensorimotor systems, including secondary cortical areas. The last areas to be myelinated are the association cortices in the frontal, parietal and temporal lobes, along with other association fibers. Along the way, the brain experiences critical periods of growth between six-eight years of age, ten-twelve years and again at 18 years of age (Fields, 2005).
Adulthood
Myelination in the brain continues during adulthood in the association areas of there brain, where integrating information for purposeful action occurs. However, despite this continued myelination, which actually peaks about age 50, the weight of the brain decreases, a process that starts as early as 20 years of age. During middle adulthood brain weight and volume decline linearly with age and is due to loss of brain mass in the white matter, cortical thinning, ventricular dilation, loss of myelin, and changes in the glial cells (Fjell & Walhovd, 2010).
Age related changes do not occur consistently throughout the brain. For example, aging affects the frontal and temporal lobes more than the parietal lobes, which is explained by a decrease in brain glucose metabolism in these areas. In addition, it appears that subcortical areas such as the thalamus, striatum and locus coeruleus are more likely to lose neurons with age. Furthermore, the hippocampus, a part of the limbic system associated with memory, has been reported to show a 30% decrease in neurons beginning as early as 30 years of age despite the fact that new neurons continue to be produced in this area. The overall decline in the total number of neurons appears to be due to the loss of pyramidal cells which play an important role in memory and learning. Fortunately, the built-in redundancy of the nervous system is such that even if neurons are lost in one place, other connections may be gained. In addition, most areas of the brainstem that deal with vital functions remain stable throughout adulthood and show minimal change with aging (Bettio, et al 2017).
In the adult, nerve conduction velocity of peripheral nerves begins to decrease. This is due to a decline in sensory nerve conduction which occurs at about 30 years of age, and a decline in motor nerve conduction velocity. After age 60 there is loss of motor neurons and myelinated anterior root fibers which contribute to the gradual loss of muscle mass and strength seen with aging.
Older Adulthood
Age related declines in brain weight and volume continue during older adulthood, although scientists are still challenged in being able to determine the extent to which this observation is due to normal aging as opposed to a pathological process. Adding to this uncertainty are observations that older adults have three conditions that are often linked to disease and dysfunction, but are also found in individuals with no known impairments or functional limitations. These conditions are lipofuscin, amyloid plaques and neurofibrillary tangles (NFT) (MorenoGarcia, 2018).
Lipofuscin is a dark, pigmented lipid found in the cytoplasm of aging neurons. Lipofuscin may be formed when there is incomplete degradation of lysosomes in mitochondria and may initially benefit the cells as a way to deal with degrading organelles. However, the amount of lipofuscin is suspected to correlate with the severity of Alzheimer’s disease, with extreme amounts of lipofuscin resulting in cell death.
Amyloid plaques, also known as neuritic plaques are another cellular hallmark aging. Plaques are comprised of a thickened mass of degenerating neuritis with an amyloid deposit in the center. They occur as a result of pathological aging and are located outside of the neuron. Neuritic plaques are most frequently found deep in the deep sulci of the neocortex, the hippocampus and the amygdala.
Neurofibrillary tangles (NFT), occurs within the perikaryon, often displacing the nucleus and distorting the cell body. The incidence of NFTs increases with normal aging in healthy brains, but it is seen in greater amounts in persons with dementia. Determining the critical point at which the presence of NFTs, neuritic plaques and lipofuscin transitions from a normal aging to pathology is still under investigation.
Reaction Time
Functionally, reaction time can be used to appreciate changes in the nervous system that occur during the normal aging process. Reaction time is defined as the amount of time between presentation of a stimulus and the motor response. It consists of three components: sensory transmission of input, central processing, and motor execution time. The combination of sensory transmission of input and central processing is called the pre-motor time and is the time between the stimulus and the onset of EMG activity. Motor time is the period from the onset of EMG activity to initiation of movement.
As would be expected, the reaction time of children is slower than that of adults. However, it develops and peaks in young adulthood. Then, reaction time remains stable for a couple of decades. A decline begins in the late 50s and 60s with a loss of 15-30%. This decline is due to changes in all three components. Both premotor and motor times are related to chronological age. Premotor time has found to be slower in all older individuals regardless of task. Motor time depends more on the type of task, especially the amount of muscular force required. Simple tasks such as hand movements show more age-related effects on the premotor time; whereas jumping shows more age-related effects on the motor time. The more complicated the task, the more likely it is to be influenced by age-related changes. The component most implicated in an adult’s decline in reaction time is central processing, which accounts for 80% of total reaction time. Slowness is related to changes in neural pathways in the brain and brainstem that are responsible for integrating sensory and motor information (Woods, et al 2015).

Figure 6.4 Active Adults
It has been demonstrated that active older adults have faster reaction times than sedentary older adults. In addition, response times have found to be faster for individuals who have engaged in exercise for long periods time over their lifespan. Nevertheless, variability among older adults is greater than typically seen in younger individuals.
Sensory Systems: Visual & Auditory
Sensory systems not only allow the brain to receive sensory stimulation, they allow for the interpretation or perception of that stimulation or sensations. So, sensation can be defined as the neural activity triggered by a stimulus that activates a sensory receptor. The result is a sensory nerve impulse sent to the brain via a sensory nerve pathway. Perception is the multi-stage process that occurs in the brain and includes selecting, processing, organizing and integrating information received from the senses. The complexity of the perception process means that perception will take more time to develop than sensation and that it will be more challenging to identify and solve problems in perception compared to impaired sensation.
Vision
Take for example, vision. The structures of the visual system allow for sight, but perception involves more than just the visual system. The expression, “Beauty is in the eye of the beholder,” illustrates how two people may view the same scene, object, or person yet have differing views or interpretations of beauty.
The are several conditions and functions of the visual system that allow for visual stimulation to be received by the brain. First, is acuity, which is the measure of visual discrimination of fine details. It is essentially the sharpness of sight. Acuity is examined using a Snellen chart during which a person stands 20 feet away from the chart and is asked to read line number 6 (see Figure 6.5) If the individual can read that line, then the visual acuity is recorded as 20/20. This value is considered to be normal. If however, a person can only read the lines 1 through 5, the score is recorded as 20/40. This score means that a person with normal acuity would be able to see line 5 from 40 feet away, rather than 20 feet away (Azzam & Ronquillo, 2021).

Figure 6.5 Snellen Chart for Visual Acuity
Within the context of visual acuity, accommodation is the process in which the curvature of the lens is changed so that objects can be clearly viewed at varying distance. This is done by the action of the ciliary muscle, which can adjust the eye’s axial length. Myopia, often referred to as near-sightedness, is when closer objects are seen clearly, but distant objects are blurred. In this situation, the axial length is too long. Hyperopia is when near objects are blurred but distant objects are seen clearly. When this occurs are person is said to be “farsighted”.

6.6 Anatomy of the Human Eye
Contrast sensitivity differs from visual acuity, yet is still an important measure of visual function, because contrast sensitivity allows for distinction between finer and finer increments of light versus dark. This is especially crucial when attempting to see with a glare or in fog, when the contrast between the objects and the background is reduced. Driving at night is an example of when contrast sensitivity is important for safety.
Visual adaptation is the ability of the eye to adjust to various levels of light and darkness. This is in part, a reflection of the pupil’s ability to adjust its diameter in response to different levels of light. An example of adaption occurs when a person first walks into a movie theater and has difficulty seeing and navigating to an open seat. After a few minutes, however, a visual adaptation to the lower light level will have occurred, decreasing the challenge of seeing things in the low light environment.
Prenatal
Prenatally, eyes begin to develop at about 4 weeks gestational age, with myelination of the optic chasm occurring at approximately 13 weeks. Rods and cones, which are the photoreceptors in the retina, begin to differentiate around 16 weeks. The rods are responsible for vision at low light levels and the cones are responsible for vision at higher light levels, as well as color vision. At approximately 20 weeks, the neurons in the occipital cortex are quite well organized, with reflexive eye blinking observed four weeks later. Thalamic connections begin to
myelinate before term and continue until the fifth postnatal month of life. These thalamic connections, are crucial for the development of visual perceptual abilities (Gaven, 2004).
Infancy through Adolescence
At the time of birth, newborns will only be able to see objects or people at about 7-10 inches away, giving them a visual acuity of 20/800. By one month of age however, the infant is expected to have 20/400 acuity. There is a debate regarding the age one should expect 20/20 vision, but most physicians will accept 20/30 vision from toddlers and preschoolers and expect that 20/20 vision will be achieved by children between the ages of 8 -10 (Almoqbel, et al, 2017).

Figure 6.7 Visual Development (0 – 6 months)
In addition to changes in acuity, a newborn will progress from seeing only black and white, to being able to see red and yellow at approximately two months of age. Full color vision is present by four months. Similar to acuity, infants are born with poor contrast sensitivity, but within the first six months, there is rapid improvement in this ability.
The first type of visual perception is noted as early as one month in which infants are able to differentiate facial features from about 20 inches away, and demonstrate a preference to look at a face rather than an object. This is sometimes referred to as face perception (Simion & Giorgio, 2015).
Smooth eye tracking abilities begin at approximately two months of age and continues to improve as the infant matures and head control is developed. Also during the 2nd month, visual accommodation develops and continues to improve until 6 – 8 months when the eye’s ability to accommodate is nearly equivalent to that of an adult.
Binocular vision is the ability to maintain visual focus on an object with both eyes, thereby creating a single visual image. When born, neonates lack binocular vision. However, at approximately three months of age, infants begin to demonstrate visual alignment and by the age of two years, exhibit adult-like binocular vision. Using the two eyes together is also important for depth perception and visual measurement of distance. During infancy, this is aided by the development of head control in the prone position as infants practice looking down.
Visual perception changes continue during childhood and adolescence. It is during this time that the integration of sensation of movement become refined. It is also the time when the individual is able to attend to more than one characteristic of a stimulus or multiple stimuli at the same time, and to use that information to plan a response. Depth perception, for example is the ability to judge the distance of objects and the spatial relationship of objects at different distances. Figure-ground perception is the ability to discriminate between the visual target, known as the figure, and the background stimuli, which is not perceived as the target. Part-to-whole perception is the ability to discriminate parts from a whole, as well as the ability to integrate the sum of the parts in a complete whole. This type of perception requires cognitive processes as well as visual stimuli.
Many aspects of visual perception develop in childhood. Children, for example, develop the ability to recognize that objects remain the same size even when the distance between them and the object changes. Figure-ground perception improves over time, such that eight year olds will have capabilities similar to an adults (Williams, 1983). Between 5 – 10 years of age, children become more accurate in their ability to track moving objects, while at the same time demonstrate improvement in depth perception. So, by the time a child is 12 years old, foundations to a catch flying disc and hit a pitched baseball are established.
Children also combine visual information with proprioception which allows them to recognize the location and orientation of objects within the environment. By age 3 – 4, most children have conceptualized the spatial dichotomies of over/under, top/bottom, front/back, and in/out.
Laterality is a conscious internal awareness of the two sides of the body. Whereas most five year old children know the right side of their body from the left side, it is not until children are approximately eight years of age that they consistently and accurately answer questions requiring right-left discrimination. Directionality, which is the motor expression of laterality and spatial awareness, also develops during childhood. This is perhaps first observed when children mirror and imitate movement. At about seven years of age, children begin to use
their body as a directional reference. For example a child may say, “The ball is in front of me.” Then, the child can reference objects objectively, such as, “The water fountain is on the left” (Long & Looft, 1972).
Adulthood and Older Adulthood
Structural changes of the eye that occur as part of the normal aging process contribute to age-related changes in function. These changes include a thickening of the lens and cornea, a decrease in the curvature of the lens, and an accumulation of a yellow pigment. In addition, functioning of the iris and pupil become impaired, such that the iris loses its ability to change width, and the pupil size remains small in both dim and bright light. In terms of function, acuity actually improves in the 20s for many people and remains fairly stable for about 20 years.
Then however, there is a decline in acuity which can rapidly increase after the age of 65. By the time an individual is 85 years of age, there is typically an 80% reduction in acuity compared to a 40 year old.
Presbyopia is the diminished ability to focus clearly at normal reading distances. One contributing cause is the thickening of the lens. In addition, the ciliary muscles, which normally act to change the curvature of the lens to focus the image become less able to adequately accommodate to distance changes; so many middle-aged and older adults complain, that their arms are “not long enough to read.” Accommodation difficulties may also impair the older person’s ability to read the speedometer in the car because of a decreased ability to change
the lens size when switching from far to near vision. Corrective lenses such as bifocals or trifocals will often become necessary (Boyd, 2016).
Color discrimination in the green-blue end of the spectrum becomes more difficult as the lens of the eye yellows with aging. Pupil size also declines with age, allowing less light into the eye. So, by 60 years of age, retinal illumination is reduced by one third, and the older adult is less able to detect low levels of light. Also, because of the lens changes, light may be scattered over more of the retinal surface, resulting in glare. Glare introduces extraneous light into the eye and may be particularly troublesome for older adults. Because of retinal sensitivity loss, an older adult’s eyes can become overstimulated by oncoming headlights or sudden flashes of light.

Figure 6.9 Older Man with Glasses
Contrast sensitivity and dark adaptation also decline with age due to a decline in the number of receptors and a decreased regeneration capacity of photoreceptor pigment. Contrast sensitivity loss is associated with impairment in depth perception, which can be especially dangerous when going up or down stairs. The use of paint or tape on the edge
of each step may help older adults accommodate for these visual impairments.
Adaptation to dim light also decreases with age. This can be hazardous when entering a darkened house or a less well illuminated room (Jackson, et al, 1999). Compared to a teenager who only needs 6 to 7 minutes to adapt to darkness, an 80-year-old may require 40 minutes. Appropriate use of night lights is often warranted so that an older adult can more safely navigate to the toilet when needed.
Impairments in non-structural aspects of the visual system can also lead to limitations in functional vision. Consider the visual evoked response (VER), which is the electro-cortical activity that occurs when there’s presentation of a visual stimulus. With aging, there is a general slowing in the latency of the VER to stimuli. The slowing appears to occur due to a decrease in the number of axons in the optic nerve, along with changes in processing in the thalamus and occipital cortex. The ocular motor system also undergoes a progressive loss of function, resulting in difficulties with convergence, upward gaze and smooth pursuit.
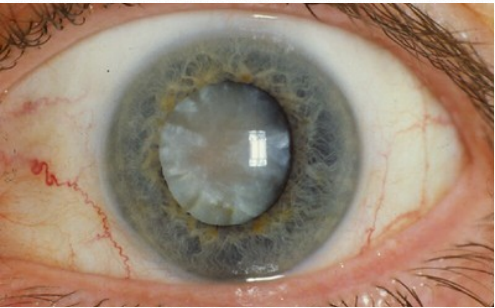
Figure 6.10 Eye with a Cataract
Visual Pathologies
Older adults are at risk for developing a few visual conditions that can result in blindness. Cataracts are opaque areas of the lens formed by the clumping together of water and protein molecules that block the light from reaching the retina. Although the rate of cataract progression can vary from person to person, fully formed cataracts are found in 33 – 50% of all persons over the age of 65. Symptoms include halos around lights, faded colors, blurring vision, double vision, poor night vision. If visual acuity is worse than 20/50, surgical removal of the
cataract is recommended and most people are very happy with the outcomes (Iroku-Malize & Kirsch, 2016).
Another pathological condition of the visual system is glaucoma. There are several types of glaucoma; however, open-angle glaucoma and closed-angle glaucoma account for more than 90% of all glaucoma cases. Although there is no cure for open-angle glaucoma, medication, laser procedures and surgical procedures can treat it effectively. Closedangle glaucoma can sometimes be a medical emergency requiring surgery called an iridotomy. Often, long-term treatment with drugs is also needed.
Macular degeneration is a degenerative disorder of the macula, an area of the eye which is rich with cone receptors and is responsible for for sharp, central vision. When the macula is damaged, the center of the field of view may appear blurry, distorted, or dark. Another sign of age-related macular degeneration (AMD) is the appearance of pigmentary changes under the retina. In some people, AMD advances so slowly that vision loss does not occur for a long time. In others, the disease progresses faster and may lead to a loss of vision in one or
both eyes. Although AMD by itself does not lead to complete and total blindness, the loss of central vision in AMD can interfere with simple everyday activities, such as the ability to see faces, drive, read, write, or do close work, such as cooking or fixing things around the house. The chance of developing AMD increases from 2% to 30% between the ages of 50 and 75 (Ehrlich, et al, 2008).
Auditory System
The auditory is responsible for the sense of hearing and can be divided into 2 subsystems. The peripheral auditory system is comprised of the outer ear, the middle ear and the inner ear. The central auditory system includes the connection between the cochlear nucleus and the primary auditory cortex. The outer ear includes the pinna, which are the folds of the ear. The pinna collects sound waves and sends them through a tube like structure called the auditory canal. Sound waves travel through the canal to the tympanic membrane, also called the
eardrum. In the middle ear, three small delicate bones called the malleus, incus, and stapes transmit the vibrations from eardrum to the cochlea, found in the inner ear. This stimulates the hair cells in the organ of Corti, which sends nervous input along the auditory portion of the vestibulocochlear nerve.
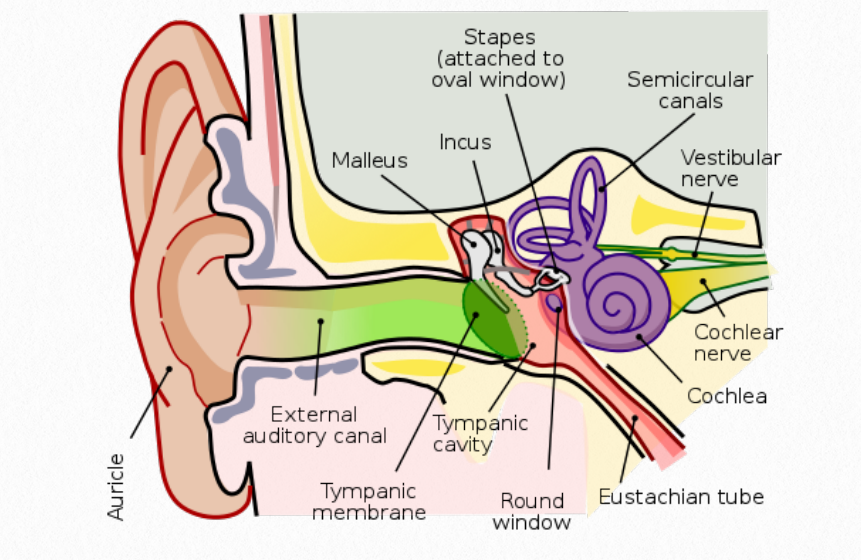
Figure 6.11 Human Ear
Signals traveling along the vestibulocochlear nerve reach different structures where the neural information is further processed. The cochlear nucleus is the first site of neural processing followed by the pons and the midbrain. Eventually, the information passes through the thalamus to the primary auditory 12 cortex of the brain, located in the temporal lobe. Here, the perception of sound including pitch and rhythm are processed (Peterson, et al, 2021).
Absolute threshold is the minimal detectable sound a hearer can sense at least half of the time a signal is sounded. Differential threshold is the closest two sounds can be presented and the hearer is able to distinguish between them at least 75% of the time.
Prenatal
In utero, the structures of the inner ear develop during the 4th week while the hair cells are differentiating by the 16th week. Fetuses appear to be able to react to sound as early as 24 weeks and hearing is consistently present after 28 weeks of gestation.
Infancy
At the time of delivery, a gelatinous material in the inner ear limits the neonate’s hearing, such that sensory thresholds are different in newborns compared to adults. The absolute threshold for a newborn is about 60 decibels higher than an adult, such that a newborn can detect an average speaking voice, whereas an adult can detect a whisper. Additionally, newly born infants do not discriminate changes in the intensity of sounds or in sound frequencies as well as adults. Fortunately, the gelatinous material is reabsorbed during the first few
weeks and hearing rapidly improves. This coincides with the myelination process, which is complete in the auditory system by approximately four weeks.
By three months of age, infants will turn their heads to localized to a sound and will be able to hear lowfrequency sounds (500-1000 hz) very well. Hearing higher frequencies (4000 hz) continues to develop and by six months, infants have hearing similar to adults. After four months, infants will search for newsounds, attempting to localize where the sound is coming from (Morrongiello, et al, 1994).
Childhood and Adolescence
Basic auditory listening skills are mastered by three years of age and auditory-evoked potentials of four year olds are similar to adults. Auditory acuity appears to improve through childhood and adolescence, but this improvement might actually be due to improvements in attention and the ability to follow directions rather than efficiencies in the auditory system.
Adults and Older Adults
Conductive hearing loss is associated with aging and can begin as early as 30 years of age and progresses until 80. Conductive hearing loss occurs when sound transmission to the inner ear is lost because the intensity of the signal is not sufficient. This may be due to impacted ear wax, perforation of the tympanic membrane or otosclerosis. Even though the signal is weakened, sound received by the inner ear can still be analyzed since the inner ear itself is not affected. Therefore, increasing the intensity of the signal through louder speech or
through mechanical amplification will assist a person’s ability to hear.
Sensorineural hearing loss occurs when there is 1) a dysfunction in the conversion of sound waves to electrical signals by the inner ear, or 2) a dysfunction in transmission of nerve impulses. Age- related sensorineural hearing loss is referred to as presbycusis and may be due 1) loss of hair cells in the organ of Corti, 2) degenerative changes in the nerve fibers of the cochlea, and/or 3) trophic changes of the cochlea. Of older adults over the age of 70, nearly 75% will exhibit some degree of this type of hearing loss (Howarth & Shone, 2006).
What happens by virtue of these impairments is that there may be a loss or decline in speech discrimination and frequency sensitivity, but not a decline in the pure-tone threshold, thus making mechanical amplification problematic. With amplification, people may be able to hear, but they are hearing unintelligible sounds. To improve communication, low-frequency tones should be emphasized. This can sometimes be addressed by turning the base up and the treble down on televisions and radios. Highfrequency devices such as doorbells, smoke alarms and telephones may need to be paired with a visual cue. To enhance communication with older adults the consider the recommendations in Figure 6.12.

Figure 6.12 Recommendations to Enhance
Communication
Final Thoughts
The nervous system is constantly adapting to both internal and external demands. All sensory systems develop in utero and have similar characteristics including transduction and coding, representation, and integration at all levels of the nervous system. For some systems such as the visual and auditory systems, additional input and time after birth allows for complete development of neural pathways. Declines in vision and hearing typically occur during the normal aging process, but neither is uniform or universal.
References
Almoqbel, F. M., Irving, E. L., & Leat, S. J. (2017). Visual acuity and contrast sensitivity development in children: Sweep visually evoked potential and psychophysics. Optometry and
Vision Science, 94(8), 830–837.
Azzam D, Ronquillo Y. Snellen Chart. [Updated 2021 May 9]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK558961/
Bettio, L., Rajendran, L., & Gil-Mohapel, J. (2017). The effects of aging in the hippocampus and cognitive decline. Neuroscience and Biobehavioral reviews, 7, 66–86. https://
doi.org/10.1016/j.neubiorev.2017.04.030.
Boyd, K. (2016). What is Presbyopia? American Academy of Ophthalmology. Bit.ly/AAOPresbyopia
Cech, D.J., & Martin, S.T. Functional Movement Development Across the Lifespan. St. Louis, MO: Elsevier: 2012.
Chugani, H. T. (2018). Imaging brain metabolism in the newborn. Journal of Child Neurology, 33(13), 851–860. https://doi.org/10.1177/0883073818792308.
Ehrlich, R., Harris, A., Kheradiya, N. S., Winston, D. M., Ciulla, T. A., & Wirostko, B. (2008). Age-related macular degeneration and the aging eye. Clinical Interventions in Aging,
3(3), 473–482. https://doi.org/10.2147/cia.s2777.
Fagard, J., Esseily, R., Jacquey, L., O’Regan, K., & Somogyi, E. (2018). Fetal origin of sensorimotor behavior. Frontiers in Neurorobotics,12, 2 3 . h t t p s : / / d o i . o rg / 1 0 . 3 3 8 9 /
fnbot.2018.00023.
Fjell, A. M., & Walhovd, K. B. (2010). Structural brain changes in aging: courses, causes and cognitive consequences. Reviews in the Neurosciences, 21(3), 187–221. https://doi.org/10.1515/revneuro.2010.21.3.187.
Fields, R.D. (2005). Myelination: An overlooked mechanismof synaptic plasticity? Neuroscientist, 11(6), 528–531. DOI: 10.1177/1073858405282304.
Graven S. N. (2004). Early neurosensory visual development of the fetus and newborn. Clinics in Perinatology, 31(2), 199– 216, v. https://doi.org/10.1016/j.clp.2004.04.010.
Howarth, A,. & Shone, G.R .(2006) .Ageing and the auditory system. Postgraduate Medical Journal, 82(965), 166-171.
Iroku-Malize, T., & Kirsch, S. (2016). Eye conditions in older adults: Cataracts. FP Essentials, 445, 17–23.
Jackson, G.R., Owsley, C., & McGwin, G. (1999). Aging and dark adaptation, Vision Research, 39(23), 3975-3982, https:// doi.org/10.1016/S0042-6989(99)00092-9.
Long, A.B., & Looft, W.R. (1972). Development of directionality in children: ages six through twelve, Developmental Psychology 6, 375–380.
Moreno-García, A., Kun, A., Calero, O., Medina, M., & Calero, M. (2018). An overview of the role of lipofuscin in age-related neurodegeneration. Frontiers in Neuroscience,
12, 464. https://doi.org/10.3389/fnins.2018.00464.
Morrongiello, B. A., Fenwick, K. D., Hillier, L., & Chance, G. (1994). Sound localization in newborn human infants. Developmental Psychobiology, 27(8),519–538. https://doi.org/ 10.1002/dev.420270805 .
Peterson DC, Reddy V, Hamel RN. Neuroanatomy, AuditoryPathway. [Updated 2021 Aug 11]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK532311/
Shiota, K., (2015). Prenatal development of the human central nervous system, normal and abnormal. Donald School Journal of Ultrasound in Obstetrics and Gynecology, 9(1), 61-
66.
Simion, F., & Giorgio, E. D. (2015). Face perception and processing in early infancy: inborn predispositions and developmental changes. Frontiers in Psychology, 6, 969. https://
doi.org/10.3389/fpsyg.2015.00969
SEER Training Modules, Nervous System. U. S. National Institutes of Health, National Cancer Institute.(Accessed Feb 9, 2022) <https://training.seer.cancer.gov/>.
Tantibanchachai, C. (2014). Teratogens. Embryo Project Encyclopedia.
Vecchierini-Blineau, M. F., & Guiheneuc, P. (1984). Motor nerve conduction velocity in children: normal values and application to a few pathologic cases. Revue D’electroencephalographie et de Neurophysiologie Clinique, 13(4), 340–348. https://doi.org/10.1016/s0370-
Welniarz, Q., Dusart, I., & Roze, E. (2017). The corticospinal tract: Evolution, development, and human disorders. Developmental Neurobiology, 77(7), 810–829. https://doi.org/
10.1002/dneu.22455.
Williams, HG. Perceptual and Motor development, Englewood Cliffs, NJ: Prentice Hall, 1982.
Woods, D. L., Wyma, J. M., Yund, E. W., Herron, T. J., & Reed, B. (2015). Factors influencing the latency of simple reaction time. Frontiers in Human Neuroscience, 9, 131. https://doi.org/10.3389/fnhum.2015.00131.
Images
Title page. [Image File]. Retrieved from https://pixabay.com/en/sound-wave-waveform-aural-audio-1781569/
Figure 6.1 Neural Plate. Retrieved from ]https://embryology.med.unsw.edu.au/embryology/images/b/b3/Neuralplate_-cartoon.png
Figure 6.2 Human Brain. Retrieved from http://www.humanbrainfacts.org/images/resource/main-img1.png
Figure 6.3 Children Jumping. Photo by Josh Mills on Unsplash, @jkmills.
Figure 6.4 Adults Exercising. Retrieved from https://www.nia.nih.gov/health/exercise-physical-activity
Figure 6.5 Snellen Chart for Visual Acuity. Drawing by Daniel
Figure 6.7 Visual Development (0 – 6 months)
Figure 6.8 Use of Vision and Perception in Motor Skills.Photo by Lori Yerdon. Retrieved from https://wordpress.org/openverse/image/e9747392-093c-4168-8477-be2419ed5c63XXXXXXXXXXXXXXX
Figure 6.9 Older Man with Glasses. Photo by Amre. Retrieved from https://wordpress.org/openverse/image/9669c085-c894-4f78-9e51-a2141e3868d7
Figure 6.10 Eye with Cataract. Retrieved from https://wordpress.org/openverse/image/edf6e223-f5e9-4b81-bf3cceae988758b6
Figure 6.11 Human Ear. Lars Chittka; Axel Brockmann, CC BY 2.5 <https://creativecommons.org/licenses/by/2.5>, via Wikimedia Commons. Retrieved from https://commons.wikimedia.org/wiki/File:Anatomy_of_the_Human_Ear.svg
Figure 6.12 Recommendations to Enhance Communication

