5 Development & Changes of the Muscular System
Development & Changes of the
Muscular System
“Just as we develop our physical muscles through overcoming opposition –
such as lifting weights – we develop our
character muscles by overcoming challenges and adversity.”
– Stephen Covey

The muscular system is comprised of skeletal, smooth and cardiac muscles and is responsible for movement of the human body including, but not limited to the arms, legs, heart and intestines. Only skeletal muscles, which number well over 600 individual muscles and account for nearly 50% of our body weight will be the focus of this chapter. Skeletal muscles are responsible for posture and the movement of the skeletal system which allow us to perform activities such sitting, standing, walking, running, reaching, grasping, and texting.
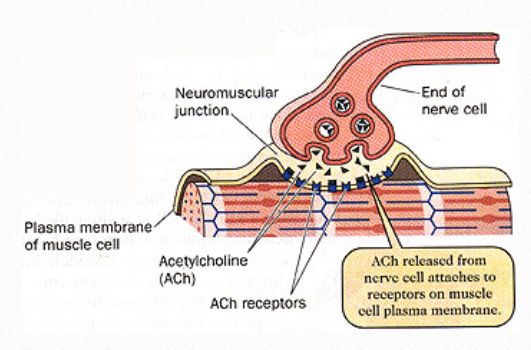
Figure 5.1 ACh release into the synaptic cleft and
attachment to receptors on the muscle
Skeletal muscles are encased by a thick sheath of collagen fibers called the epimysium, which extends into the muscle and subdivides the muscle into myofibers called fascicles. Within each fascicle, individual myofibers are encased in a sheath of connective tissue called endomysium. All the coverings come together at the tendinous junction where the contractile forces of the muscle are transferred to bone via the tendinous attachment (SEER training modules, 2022). Not all muscles however are attached to bone. Some skeletal muscles are attached to other muscles or skin, responsible for actions such as a smile and a wink. Regardless of their attachment, muscle fibers are mostly made up of myofibrils, which are comprised of active proteins and are arrange into repeating sub-units called sarcomeres. The sarcomeres, which are the actual functional unit of the muscle fiber, include a thick protein filament called myosin and actin, a thin filament. Other proteins include tropomyosin, which wraps around actin and troponin, and acts to move tropomyosin away from the myosin binding sites
found on the actin filaments (Geeves, 1991).
For a skeletal muscle contraction to occur, a motor neuron receives input from the central nervous system and carries an action potential to the muscle at the motor endplate. Acetylcholine (ACh) is released into the synaptic cleft and binds with receptors on the cell membrane of the muscle fiber (Figure 5.1). This opens the calcium channels and calcium is released.
Calcium then binds to troponin which shifts the tropomyosin, allowing the myosin to form a crossbridge on the actin. The cross bridge pulls the actin inward. The action requires energy, which is provided by adenosine triphosphate (ATP), a nucleotide often referred to as the energy currency of life. ATP binds to the myosin, and breaks down the actinmyosin cross-bridge. This process causes shortening of the sarcomere and the result is a muscle contraction (Fig 5.2) (Kuo & Ehrlich, 2015)
Muscle fibers are most often characterized as either slow twitch (Type I) or fast twitch (Type II). Fast twitch fibers can be further identified as Type IIa and Type IIb. Type I fibers fire more slowly than Type II fibers and are more efficient at using oxygen and generating ATP, which provides the energy needed for extended muscle contractions over a long period of time. Type II fibers use anaerobic metabolism for energy and are able to fire more rapidly than Type I fibers, as a result they fatigue more quickly. The Type IIb fibers have the highest rate of contraction and are especially efficient at producing quick powerful bursts of speed, but require quickly require rest. The Type IIa fibers can use aerobic and anaerobic metabolism to create energy and are considered to be a combination of Type I and Type II muscle fibers (Talbot & Maves, 2016).

Figure 5.2 Actin and myosin during muscle contraction
Prenatal
Nearly all skeletal muscles are present at 8 weeks of gestational age, which is the end of the embryonic period. Muscle development (myogenesis) begins with the primary myotubes which are developed from embryonic myoblasts. These myoblasts can be differentiated into Type I and Type II fiber types. In general most primary myotubes become Type I fibers and secondary myotubes most often become Type II fibers, requiring neural input for development and differentiation. There are also differences in prenatal muscle fibers due to the different forms of sarcomeric proteins present during the fetal period (Brown, 2014).
Muscle fibers initially develop with a cranio-caudal orientation and then migrate to become aligned based on an individual muscle’s origin, insertion and architectural structure. In a multipennate muscle, such as the deltoid, muscle fibers run obliquely or diagonally from a tendon of origin to a tendon of insertion. A muscle that has maintained a cranio-caudal orientation is the biceps brachii, which is classified as fusiform muscle because its fibers are spindle shaped and run in parallel with each other.
During the last half of the gestational period, there is a remarkable increase in the number and size of muscle fibers, largely due to an increase in Type II fibers. Regardless of the type of fiber however, muscle growth during the prenatal period is by hyperplasia and hypertrophy. Hyperplasia is an absolute increase in the number of muscle cells, where
as hypertrophy is an increase in the relative size of the cells. Although hyperplasia does continue after birth, post-natal muscle growth occurs predominately by hypertrophy and is influenced by functional movements, activities and exercise (Brown, 2014).
Infancy and Childhood
At birth, muscle accounts for approximately 25% of a baby’s weight. During infancy and childhood muscle fibers continue to differentiate and will typically reflect their functional use. In early infancy, the slow-twitch characteristics of postural muscles have not yet developed, so they have difficulty with head and trunk control. Their muscle responses are
most often rapid, with reflexive movements of their arms and legs. This can be characterized as fast twitch movements. Then, the postural muscles begin to develop adult-like slow-twitch characteristics, so that during childhood, these muscle have completed their differentiated development and can be used to maintain postures and provide the muscular support for balance (Cech & Martin, 2012).
Also during childhood are changes in contractile properties of skeletal muscles. Researchers have found that children’s muscles are initially slow to relax after a contraction, but that relaxation speed doubles between 3 and 10 years of age (Gatev, et al.,1977). As the muscle is able to relax more quickly it is also able to increase it’s rate of contraction.
By the age of 10, most children demonstrate contraction and relaxation speeds similar to adults.
Figure 5.3 Child looking tough with muscles
Adolescence and Adulthood
Muscles that primarily act as fast-twitch muscles because of their functional demands and use, do not exhibit adult proportions of Type II fibers until young adulthood, suggesting that muscle fibers continue to differentiate throughout childhood and adolescence. Puberty also stimulates other changes in musculoskeletal system. As bones get longer, muscles need to lengthen in order to re-establish appropriate length-tension relationship. The resting length of a muscle affects the amount of tension that can be generated, so when bones grow, muscle fibrils and sarcomeres are added in order for a new muscle length can be achieved. (Cech & Martin, 2012). Greater muscle growth and mass is seen in males compared to females due to the effects of steadily rising levels of testosterone. Growth hormone, insulin and the thyroid hormones are also important for muscle growth and it’s through those hormones that girls gain strength, especially during adolescence. Muscle differences between boys and girls can also be attributed to the fact that males tend to gain more height over a longer period of time than females, providing them more of an opportunity for muscle development (Gillen, et al, 2019). Between the ages of 5 and 17, the percentage of muscle mass typically increases from 25% at birth to approximately 40% in females, whereas males can expect muscle mass to increase to 50-53%. Strength, which is a function of muscle mass, peaks during young adulthood. By the late thirties, muscle mass declines by at least 5% in sedentary individuals and continues to decline at an accelerated rate throughout adulthood. Pronounced changes occur after 50 years of age with a 15% loss of strength documented per decade (Keller & Engelhardt, 2013). Figure 5.4 shows the relationship between age and maximal isometric muscle
strength in healthy adults along with factors that contribute to a decline of strength during the aging process.

Figure 5.4 Relationship between age and maximal isometric muscle strength
Older Adulthood
During later and older adulthood, muscle mass and strength continues to decline. For active individuals, especially those who participate in resistance training activities, muscle mass and strength can be preserved somewhat, but aging’s negative effect on skeletal muscle cannot be prevented.
Sarcopenia is muscle loss associated with the aging process. It is thought to be due to impaired protein synthesis that results in a loss of contractile proteins such as actin and myosin. In addition, the aging process is associated with a decline in circulating hormones, many of which play a role in the maintenance of muscle mass, including growth hormone, testosterone, insulin-like growth factor and DHEA. This results in a decrease in the number of muscle fibers within a muscle as well as a decrease in size of individual muscle fibers (Sakuma & Yamaguchi, 2012).
Muscle weakness that occurs during the aging process is also associated with changes in the nervous system which results in a decline in the total number of motor neurons and motor units. In addition, it appears that there is a selective denervation of Type II fibers with aging. A greater loss of fast twist muscle fibers relative to slow twitch fibers, results in a faster decline in power than a decline in strength and appears to affect women more than men (Deschenes, 2004).
Changes across multiple systems including the cardiovascular, pulmonary, endocrine systems combined with alterations of the neuromuscular system account for relative declines in strength and power during older adulthood. It is important to note that the aging process has a greater negative effect on concentric contractions and dynamic strength that I is does on eccentric contractions and isometric strength.

Figure 5.5 Muscle contraction changes
Older adulthood is often associated with endurance impairments and complaints of fatigue. Although these are experienced in similar ways, appreciation of the subtle differences between the mechanisms of endurance and fatigue is important. Endurance is related to the aerobic capacity to muscle, whereas fatigue in an older adult may be the result of failed
motor unit recruitment or altered excitation-contraction coupling (Baudry, et al., 2007). Thus, muscle fatigue does not result from aerobic capacity alone. Rather, it occurs at least in part due to changes in the central and peripheral nervous systems.
The good news is that endurance and strength training can significantly increase older adults’ functional capacity. Endurance training has been demonstrated to improve mitochondrial oxidative capacity in older adults, and progressive resistance exercises result in significant muscle strength gains as well as improvement in functional activities such as sit to stand, ambulation and stair climbing (Liu & Latham, 2009). Even the oldest old demonstrate the capacity to gain strength and improve functional mobility (Fiatarone, et al., 1994).
Final Thoughts
Genetics, nutrition, overall health and activity level can influence pre- and post-natal muscle development. Muscle maturation occurs throughout childhood and strength continues to develops through adolescence. The decline of strength that occurs in middle adulthood become even more remarkable after the age of 50. Muscle mass loss, known as
sarcopenia, negatively affects strength and muscle mass and plays a role in declining functional abilities. Although age related changes in the muscular system cannot be stopped, they can be slowed through appropriately applied resistance exercise and endurance training.
“We can’t avoid age. However, we can avoid
some aging. Continue to do things. Be active.
Life is fantastic in the way it adjusts to demands;
if you use your muscles and mind, they stay
there much longer.”
-Charles H. Townes
References
Baudry, S., Klas, M., Pasquet, B., et al. (2007). Age-related fatigability of the ankle dorsiflexor muscles during concentric and eccentric contractions. European Journal of Applied Physiology, 100, 515-526.
Brown L. D. (2014). Endocrine regulation of fetal skeletal muscle growth: impact on future metabolic health. The Journal of Endocrinology, 221(2), R13–R29 https://doi.org/
10.1530/JOE-13-0567
Cech, D. J. & Martin, S. T. Skeletal System Changes. In D.J. Cech, & S.T. Martin (Eds.), Functional Movement Development Across the Life Span. St. Louis, MO, Elsevier: 2012.
Deschenes, M.R., Effects of aging on muscle fibre type and size. Sports Medicine, 34(12), 809-824.
Fiatarone, M.A., O’Neill, E.F., Ryan, N.D., et al. (1994). Exercise training and nutritional supplementation for physical frailty in very elderly people. New England Journal of Medicine, 330, 1769-1775.
Gatev, V., Stamatova, L., & Angelove, B. (1977). Contraction time in skeletal muscles of normal children. Electromyography in Clinical Neurophysiology, 17, 441-452.
Geeves M. A. (1991). The dynamics of actin and myosin association and the crossbridge model of muscle contraction. The Biochemical Journal, 274 (Pt1)(Pt1),1–14.https://doi.org/
10.1042/bj274000.
Gillen, Z. M., Shoemaker, M. E., McKay, B. D., Bohannon, N. A., Gibson, S. M., & Cramer, J. T. (2019). Muscle strength, size, and neuromuscular function before and during adolescence. European Journal of Applied Physiology, 119(7), 1619–1632. https://doi.org/10.1007/s00421-019-04151-4
Keller, K., & Engelhardt, M. (2013). Strength and muscle mass loss with age process. Age and strength loss. Muscles, Ligaments and Tendons Journal, 3(4), 346-350.
Kuo, I. Y., & Ehrlich, B. E. (2015). Signaling in muscle contraction. Cold Spring Harbor Perspectives in Biology, 7(2), a006023. https://doi.org/10.1101/cshperspect.a006023
Liu, C.J, & Latham, N.K. (2009). Progressive resistance strength training for improving physical function in older adults. Cochrane Database of Systematic Reviews, Issue 3. Art. No.: CD002759. OI: 10.1002/14651858.CD002759.pub2
Sakuma, K., & Yamaguchi, A. (2012). Sarcopenia and age related endocrine function. International Journal of Endocrinology, 2 0 1 2 , 1 2 7 3 6 2 . h t t p s : / / d o i . o r g /
10.1155/2012/127362
SEER Training Modules, Introduction to the Muscular System. U. S. National Institutes of Health, National Cancer Institute. (Feb. 8, 2022) <https://training.seer.cancer.gov/>.
Talbot, J., & Maves, L. (2016). Skeletal muscle fiber type: using insights from muscle developmental biology to dissect targets for susceptibility and resistance to muscle disease.
Wiley Interdisciplinary Reviews. Developmental Biology, 5(4), 518–534. https://doi.org/10.1002/wdev.230
Tonson, A., Ratel, S., Le Fur, Y., et al (2008). Effect of maturation on the relationship between muscle size and force production. Medicine and Science in Sports and Exercise, 40(5),
918-925.
Images
Title page. Muscular System [Image File]. Retrieved from: http://arabic.sport360.com/wp-content/uploads/2017/11/muscle-contraction-rolfing.jpg
Figure 5.1 Release of acetylcholine (ACh) at the synaptic cleft. http://www.physioweb.org/muscular/contraction.html
Figure 5.2 Actin and myosin during muscle contraction http://www.physioweb.org/muscular/contraction.html
Figure 5.3 Child looking tough with muscles. Image from “Media from Wix”
Figure 5.4 Relationship between age and maximal isometric
muscle strength. From Cech, D. J. & Martin, S. T. Skeletal In D.J. Cech, & S.T. Martin (Eds.), Functional Movement Development Across the Life Span. St. Louis, MO, Elsevier:
2012. Modified from Vandervoot, A.A., Biological and Physiological changes. I Pickles, B., Campton, A. et al, (Eds). Physiotherapy with Older People, Philadelphia, PA: WB
Saunders, 1995.

